The Promise of Factor XI
Hiding in Plain Sight:
Factor XI as a Potentially Attractive Therapeutic Target
It has long been believed that reducing thrombosis (harmful blood clotting) and hemostasis (healthy blood clotting) are so closely connected that reducing clots would always cause bleeding. Therefore, bleeding is often seen as an unavoidable consequence of anticoagulant therapy.
Despite significant advances in anticoagulation therapy during the past decade, balancing the prevention of thromboembolism with the potential tradeoff of increased bleeding risk remains a major concern for both clinicians and patients.
New Hope for Patients has Emerged
Epidemiologic and genetic data suggest that individuals with congenital FXI deficiency benefit from a lower lifelong risk of ischemic stroke and venous thromboembolism compared to the general population.1
A recent study found that people with FXI deficiency were nearly half as likely to have a cardiovascular event (stroke, transient ischemic attack, and myocardial infarction/heart attack) and even less likely to develop a venous thromboembolism (VTE) compared to those with normal Factor XI levels.2
Factor XI (FXI) has emerged as a promising target for anticoagulants, supported by mounting evidence that FXI is crucial for thrombosis but is not necessary for normal, healthy clotting (or hemostasis).3,4
APCC, activated prothrombin complex concentrate; FFP, fresh frozen plasma; FVIIa, activated factor VII; FXI, factor XI; IV, intravenous; SC, subcutaneous.
Source: Fredenburg JC, Gross PL, Weitz JI. Emerging anticoagulant strategies. Blood 2017; 129:147-54.
Abelacimab is an investigational agent and is not approved for any indication in any country
Abelacimab Clinical Development Program
Abelacimab:
A Novel Anticoagulant
Over the past decade, efforts to develop safer antithrombotic strategies have largely focused on Factor XI.5
Abelacimab is a novel, highly selective, fully human monoclonal antibody that binds tightly to Factor XI to block its activation and prevent the generation of the activated form (Factor XIa).5 This mimics natural Factor XI deficiency, which is associated with protection from thromboembolic disease.6
As a fully human monoclonal antibody, abelacimab is not metabolized via the cytochrome P450 system or as a substrate for P-glycoprotein, meaning the risk for drug-drug interactions is very unlikely.4 There is also no need to adjust the dose based on age or renal/hepatic status4 and it can be safely co-administered with antiplatelet agents.7
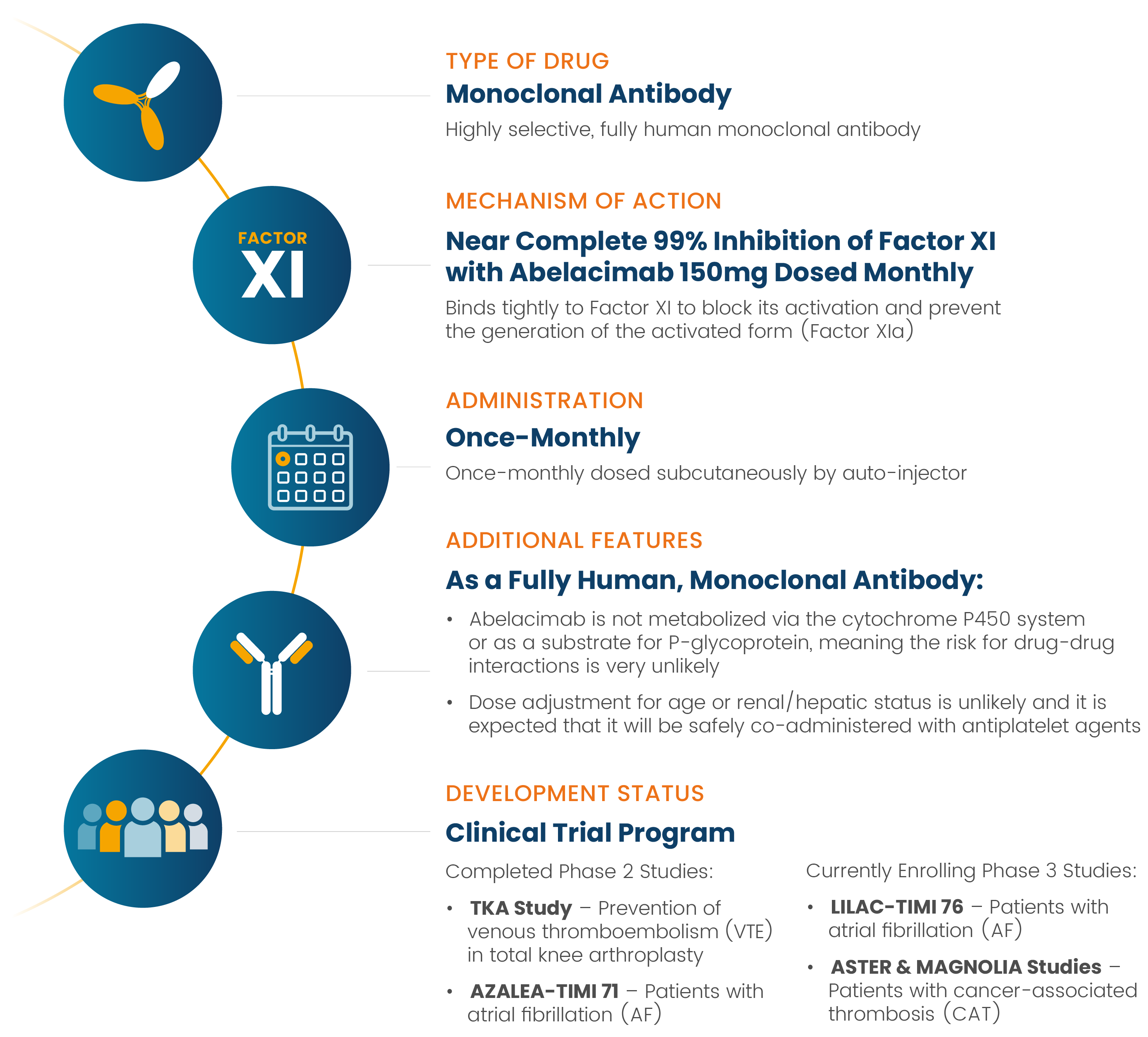
Abelacimab is currently being evaluated in several ongoing Phase 3 clinical trials for patients at risk of dangerous arterial and venous clots, including those with atrial fibrillation and cancer associated thrombosis.
In patients with atrial fibrillation, abelacimab is planned to be dosed subcutaneously once-monthly with an autoinjector to maintain near-complete inhibition in a chronic setting. It is also planned to be administered via an initial intravenous (IV) infusion for acute indications requiring immediate onset of action and then followed by subsequent monthly subcutaneous administration.
Abelacimab received a Fast Track Designation from the FDA in July 2022 for the treatment of thrombosis associated with cancer and, in September 2022, a second Fast Track Designation for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Abelacimab is an investigational agent and is not approved for any indication in any country
1. Weitz JI, Fredenburgh JC. Front Med 2017; 4:19. 2. Preis M et al. Blood 2017;129(9):1210–5. 3. Koch AW et al. Blood 2019;133(13):1507-1516. 4. Hsu C et al. J Am Coll Cardiol 2021;78(6):625-631. 5. Gailani D, Gruber A. Blood. 2024 Apr 11;143(15):1465-1475. 6. Goodman SG et al. Crit Pathways in Cardiol 2024;23: 47–57. 7. Khder Y et al. Abstract PB0927. ISTH, 2022.
