Abelacimab Clinical
Development Program
Abelacimab is an investigational agent and is not approved for any indication in any country
Abelacimab for Prevention of Venous Thromboembolism
Published in the New England Journal of Medicine 1
This Phase 2 proof-of-concept study demonstrated that a single postoperative dose of abelacimab reduced the rate of venous thromboembolism (VTE) by ~80% compared to enoxaparin (a commonly used anticoagulant), following elective total knee arthroplasty (TKA), the gold standard setting for potential new anticoagulants to demonstrate efficacy.
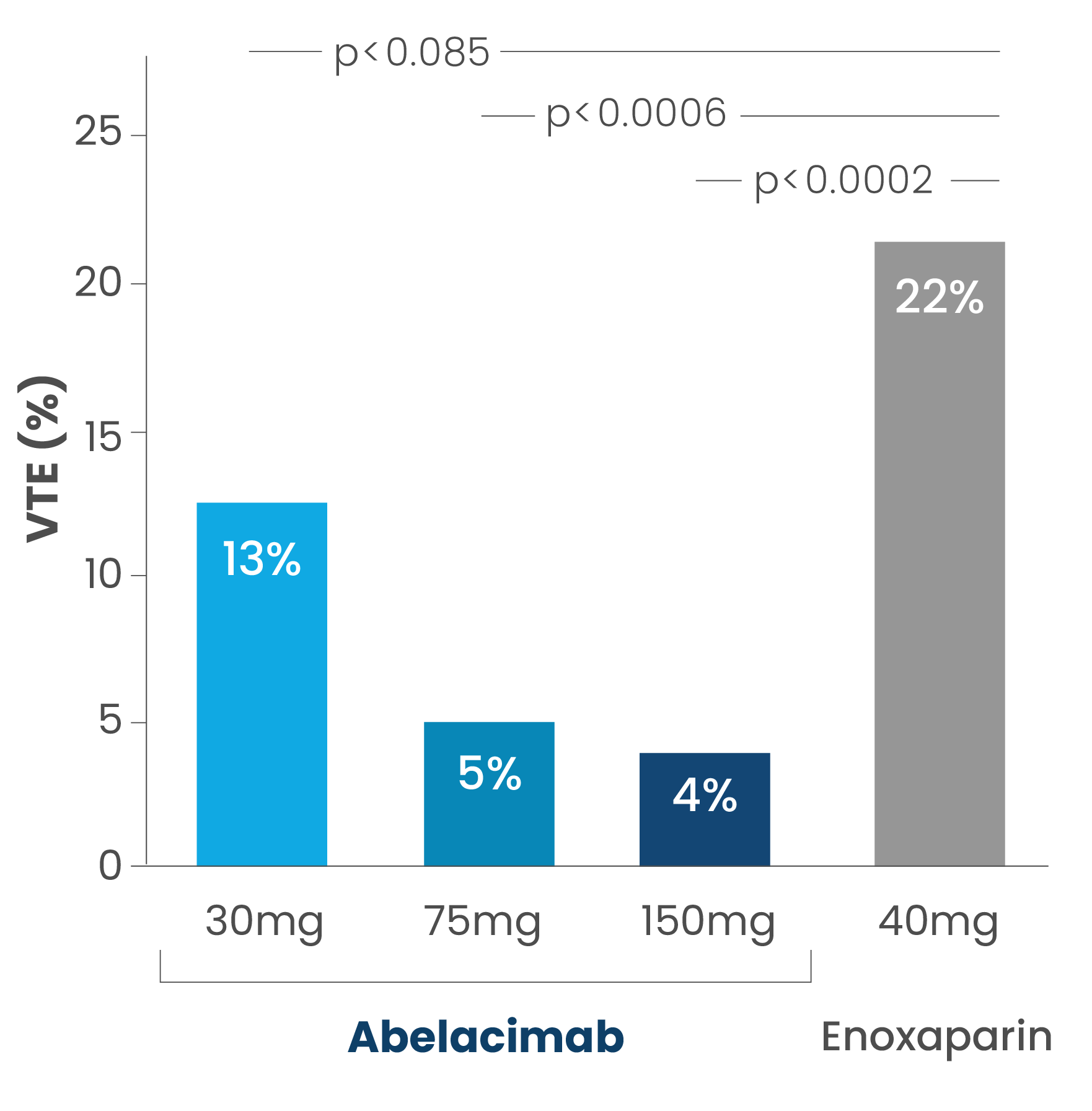
In this parallel-group study, 412 participants were randomly assigned to one of three single postoperative intravenous doses of abelacimab (150mg, 75mg or 30mg) in a blinded fashion or open-label standard of care enoxaparin given subcutaneously 40mg once daily for approximately 10 days after surgery.
The primary composite efficacy outcome – which included deep vein thrombosis detected by venography of the operated leg and documented symptomatic VTE events – occurred in 4%, 5% and 13% of patients in the 150mg, 75mg and 30mg abelacimab groups respectively, compared with 22% of patients in the enoxaparin group. The 75mg and 150mg abelacimab regimens were both statistically superior to enoxaparin (p<0.001) while the 30mg dose was non-inferior. Abelacimab was well tolerated with no safety signals and bleeding was insignificant in both study arms.
Anticoagulant drug development has typically included Phase 2 dose-ranging studies in patients undergoing total knee arthroplasty, a population at high risk for venous thromboembolism, to demonstrate proof-of-concept that agents reduce thrombosis and to guide dose selection. However, not all Factor XI inhibitors in Phase 3 clinical development have performed such a study. The demonstration of efficacy and safety in this patient population has traditionally translated into efficacy across other clinical indications including stroke prevention for AF.2
Rates of venous thromboembolism (VTE) with abelacimab vs enoxaparin
About the Atrial Fibrillation Clinical Trial Program

AZALEA-TIMI 71 Study
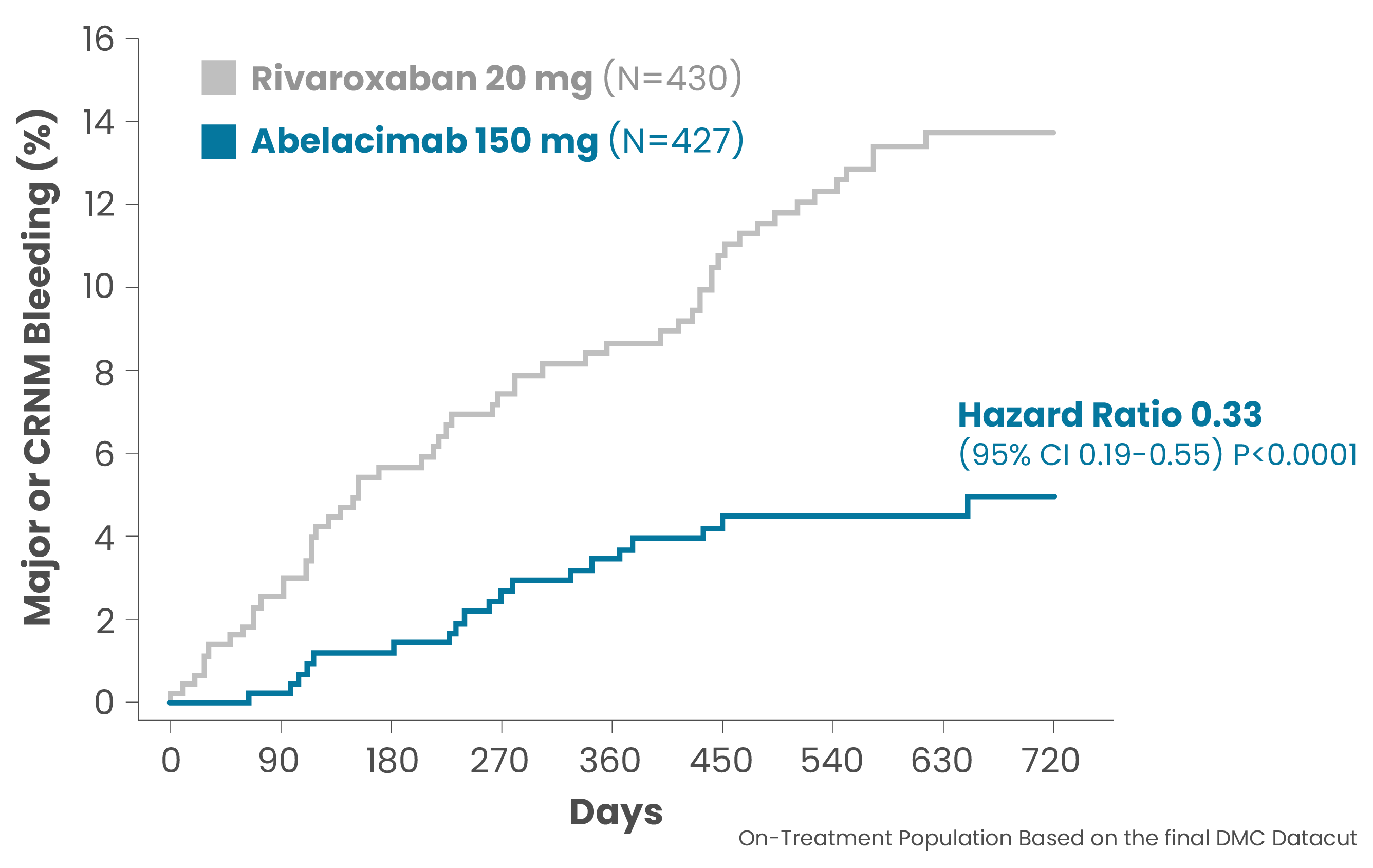
The AZALEA-TIMI 71 Phase 2 study is an event-driven, randomized, active-controlled, blinded endpoint, parallel-group study with a primary endpoint that evaluated the effect of two blinded doses of abelacimab relative to open-label rivaroxaban in patients with atrial fibrillation (AF) who are at moderate-to-high risk of stroke. The primary endpoint of the AZALEA-TIMI 71 study was the composite of the rate of major or clinically relevant non-major bleeding events. Patients were randomized 1:1:1 and administered subcutaneous (SC) abelacimab 150mg once-monthly, abelacimab 90mg once-monthly, or rivaroxaban 20mg daily.
With a median follow-up of 21 months, the AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to a standard-of-care anticoagulant.
The AZALEA-TIMI 71 study enrolled 1,287 patients across 95 global study sites including the U.S. and Canada, Europe and Asia. The independent data monitoring committee (IDMC) recommended that the main study end early because of a substantially greater than anticipated reduction in major and clinically relevant non-major bleeding in the abelacimab arms compared to rivaroxaban and a benefit: risk ratio that favored abelacimab. At the same time, the IDMC also recommended that an optional open-label extension period should be made available.
Study Results
The initial results of the AZALEA-TIMI-71 study in patients with atrial fibrillation at moderate-to-high risk of stroke were presented at the 2023 American Heart Association’s Scientific Congress during a late-breaking session. For the primary endpoint, abelacimab 150mg, dosed once-monthly, demonstrated a highly significant 67% reduction in major or clinically relevant non-major bleeding. In addition, there was a substantial 74% reduction in major bleeding alone and a 93% reduction in gastrointestinal (GI) bleeding, all favoring abelacimab over rivaroxaban, a standard-of-care anticoagulant.
Primary End Point
Summary of Presented Results
- Across all bleeding endpoints, abelacimab demonstrated a highly significant reduction versus rivaroxaban
- Factor XI inhibition of ~99% with abelacimab 150mg dosed once monthly
- Abelacimab 150mg dosed once monthly via subcutaneous injection has been selected for the Phase 3 clinical study program
- Primary endpoint met with a 67% reduction in major or clinically relevant non-major bleeding (CRNM) with abelacimab 150mg compared with rivaroxaban 20mg in patients with atrial fibrillation who are at moderate-to-high risk of stroke (P<0.0001, HR 0.33, 95% Cl 0.19–0.55).
Other Bleeding Endpoints
- 74% reduction in major bleeding alone with abelacimab 150mg vs rivaroxaban 20mg (P=0.002, HR 0.26, 95% CI 0.11-0.61)
- 93% reduction in gastrointestinal (GI) bleeding with abelacimab 150mg vs rivaroxaban 20mg (P=0.008, HR 0.07, 95% CI 0.01-0.50)
Exploratory Endpoint
- ~50% improvement in net clinical outcome favoring abelacimab 150mg vs rivaroxaban (P<0.001, HR 0.49, 95% Cl 0.33-0.71) *Net clinical outcome is a composite endpoint of ischemic stroke, systemic embolism, bleeding (major or CRNM), and all-cause death
Long-acting Factor XI Inhibition and Periprocedural Bleeding
A Secondary Analysis from AZALEA-TIMI 71
An estimated 20% of patients treated with an anticoagulant undergo invasive procedures per year, with frequent interruption of therapy.3,4 Thus, the perioperative management of therapies is a common scenario encountered among patients with atrial fibrillation treated with anticoagulants for stroke prevention.
A new analysis from the landmark AZALEA-TIMI 71 study, demonstrated that approximately 1% of patients randomized to abelacimab, an investigational Factor XI inhibitor, experienced a major or clinically relevant non-major procedural bleed while undergoing invasive procedures.
- 1,287 patients followed for a median of 2.1 years
- 920 invasive procedures occurred (34% in abelacimab arms vs. 36% for rivaroxaban)
- Standard-of-care approach allowed for interruption of rivaroxaban 24-48 hours prior to procedures
Routine interruption of anticoagulation may not be necessary in advance of all invasive procedures with abelacimab. Ongoing phase 3 trials are required to further determine the best strategy depending upon the type of procedure being performed.
AZALEA-TIMI 71 Open-Label Extension
When the independent data monitoring committee (IDMC) stopped the AZALEA-TIMI 71 study early due to significantly lower bleeding rates favoring abelacimab over rivaroxaban, they recommended an open-label extension for all eligible patients to benefit from abelacimab treatment. Investigative sites could choose whether to participate in this extension. Approximately 84% of eligible patients opted to transition to abelacimab, including 75% of patients in the rivaroxaban arm who voluntarily switched from the once-daily oral anticoagulant to once-monthly abelacimab.

LILAC-TIMI 76 Study
The LILAC-TIMI 76 Phase 3 study is an event-driven, randomized, placebo-controlled, double-blind, parallel-group study. It aims to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in patients with atrial fibrillation (AF) who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients in the study will be randomized to receive abelacimab 150mg subcutaneous (SC) or matching placebo once monthly. The study is targeting to enroll approximately 1,900 patients from more than 400 sites in North America, United Kingdom, Europe, India, Latin America, the Middle East and Asia. Abelacimab received FDA Fast-Track designation for the prevention of stroke and systemic embolism in patients with atrial fibrillation in September 2022.
Prospective Study Participants
Atrial fibrillation patients who may be good candidates to participate in the LILAC-TIMI 76 Phase 3 study include those who:
- are at a higher risk of bleeding
- are 65 years of age or older
- have multiple co-morbidities
- are taking multiple medications
- have renal or hepatic impairment
- are taking anti-platelet agents
LILAC-TIMI 76 Enrollment
Healthcare practitioners interested in potentially enrolling patients should email LILACTIMI76@anthostherapeutics.com to connect with a member of our LILAC-TIMI 76 study team.
About the Cancer Associated Thrombosis (CAT) Program
The Phase 3 CAT program comprises two complementary studies targeting to enroll approximately 2,700 patients across 220 sites in more than 23 countries – the largest program of any anticoagulant performed in cancer associated thrombosis. Abelacimab received its first Fast Track Designation from the FDA in July 2022 for the treatment of thrombosis associated with cancer.

ASTER Study
The ASTER study compares the effect of abelacimab relative to apixaban on venous thromboembolism (VTE) recurrence and bleeding in patients with cancer-associated VTE in whom direct oral anticoagulant (DOAC) treatment is recommended.

MAGNOLIA Study
The MAGNOLIA study compares the effect of abelacimab relative to dalteparin in patients with gastrointestinal (GI)/genitourinary (GU) cancer in whom direct oral anticoagulant (DOAC) treatment is not recommended.
Abelacimab is an investigational agent and is not approved for any indication in any country
