Anthos Therapeutics’ Novel Dual Acting Factor XI / XIa Inhibitor, Abelacimab 150 mg, Demonstrated a 67% Reduction in the Primary Endpoint of Major or Clinically Relevant Non-Major Bleeding Compared with Rivaroxaban in Patients with Atrial Fibrillation
Secondary Endpoints Also Showed a Highly Significant 74% Reduction in Major Bleeding and a 93% Reduction in Gastrointestinal (GI) Bleeding with Abelacimab
These Results Triggered the Data Monitoring Committee (DMC) to Stop the AZALEA-TIMI 71 Study Early Due to a Substantially Greater Than Anticipated Reduction in Bleeding with Abelacimab Compared to Rivaroxaban
The AZALEA-TIMI 71 Study is the Largest and Longest Head-to-Head Study of a Factor XI inhibitor vs. a Direct Oral Anticoagulant (DOAC) Completed to Date
Abelacimab is a Once-Monthly, Highly Selective, Fully Human Monoclonal Antibody with Dual Activity that Achieves a Near Complete 99% Inhibition of Factor XI and its Active Form, Factor XIa
As a Monoclonal Antibody, Abelacimab Does Not Require Dose Adjustments Based on Renal or Hepatic Status and is not Constrained by any Drug-Drug Interactions
CAMBRIDGE, Mass., November 12, 2023 (BUSINESS WIRE) – Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for cardiovascular diseases, founded by Blackstone Life Sciences, announced today during a Late-Breaking session of the American Heart Association (AHA) meeting that abelacimab demonstrated a highly significant reduction in bleeding events across all primary and secondary endpoints versus a standard of care direct-oral anticoagulant (DOAC). With a median of 21 months of follow-up spanning more than 2,000 patient-years, the AZALEA-TIMI 71 study evaluated the safety and tolerability of abelacimab as compared with rivaroxaban in 1,287 patients with a moderate to high risk of ischemic stroke. Anthos Therapeutics has initiated an extension study to enable patients to transition from rivaroxaban to abelacimab to benefit from the improved bleeding profile.
Atrial fibrillation, also known as AF, or AFib, is an irregular heartbeat, or arrhythmia which can lead to blood clots, stroke, heart failure and other heart-related complications.1 Approximately 37 million people worldwide are currently diagnosed with atrial fibrillation,2 while in the United States, the Centers for Disease Control and Prevention (CDC) estimates that more than 12 million Americans will develop AF by 2030.3 The goal of anticoagulation therapy is to prevent and or treat thrombosis with minimal disruption of hemostasis. Although currently available anticoagulants are effective in preventing strokes in patients with atrial fibrillation, the risk of bleeding remains an issue.
“With a substantial 67% lower risk of bleeding for the combined primary endpoint of major or clinically relevant non-major bleeding, these ground-breaking results not only support the superior safety of abelacimab as compared with a commonly used DOAC, but also strongly supports the promise of Factor XI inhibition as a paradigm-shifting treatment approach,” said Christian T. Ruff, MD, MPH, Principal Investigator, AZALEA-TIMI 71, Director, General Cardiology, Brigham and Women’s Hospital, Associate Member, Broad Institute of MIT and Harvard, Associate Professor of Medicine, Harvard Medical School. “The unfortunate reality today is that too many people who could benefit from DOACs are unable to do so because of a host of clinical challenges, with bleeding or the fear of it, at the top of the list. We are hopeful that abelacimab’s remarkably improved bleeding profile and its unique attributes as a once monthly fully human monoclonal antibody with no renal clearance or drug-drug interactions, will help address many of the issues that patients face today.”
Overview of Study Results
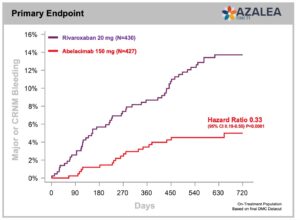
Summary of Presented Results
- Across all bleeding endpoints, abelacimab demonstrated a highly significant reduction versus rivaroxaban
- Factor XI inhibition of ~99% with abelacimab 150 mg dosed once monthly
- Abelacimab 150 mg dosed once monthly via subcutaneous injection has been selected for the Phase 3 clinical study program
- Primary endpoint met with a 67% reduction in major or clinically relevant non-major bleeding (CRNM) with abelacimab 150 mg compared with rivaroxaban 20 mg in patients with atrial fibrillation who are at moderate-to-high risk of stroke (P<0.001, HR 0.33, 95% Cl 0.19–0.55).
Other Bleeding Endpoints
- 74% reduction in major bleeding alone with abelacimab 150 mg vs rivaroxaban 20 mg. (P=0.002, HR 0.26, 95% CI 0.11-0.61)
- 93% reduction in gastrointestinal (GI) bleeding with abelacimab 150 mg vs rivaroxaban 20 mg (P=0.008, HR 0.07, 95% CI 0.01-0.50
“With dual inhibitory activity against both Factor XI and XIa, abelacimab 150 mg has been proven to maintain approximately 99% inhibition over the monthly dosing interval. By providing patients with the possibility of continuous protection against blood clots, the potential for enhanced adherence and a placebo-like bleeding profile, abelacimab could represent the ‘holy grail’ of hemostasis-sparing anticoagulation,” said Dan Bloomfield, MD, Chief Medical Officer of Anthos Therapeutics. “Based on the evidence to date, we are highly confident that abelacimab will offer a potential game- changing treatment approach for patients with atrial fibrillation.”
In patients with atrial fibrillation, suboptimal DOAC adherence is associated with poor clinical outcomes and a 39% higher hazard of stroke and increased risk of thromboembolic events and all-cause mortality. Due to their short elimination half-lives and short duration of anticoagulant effect, even brief periods of DOAC nonadherence may rapidly result in subtherapeutic anticoagulant levels.4
In September 2022, the Food and Drug Administration (FDA) granted abelacimab Fast Track Status for the prevention of stroke and systemic embolism in patients with atrial fibrillation recognizing the important unmet medical need and the potential benefits of abelacimab. The Fast Track Designation process is intended to facilitate the development and expedite the review of treatments for serious medical conditions, with the hope of getting important new drugs to the patient earlier.5
“We are very optimistic about the potential future for AFib patients after seeing the very impressive reduction that abelacimab demonstrated versus a standard of care anticoagulant in the AZALEA-TIMI 71 study,” said Mellanie True Hills, Founder and Chief Executive Officer of the nonprofit patient advocacy organization StopAfib.org. “In a recent large patient survey, a considerable number of AFib patients reported bleeding and/or bruising problems since starting an anticoagulant that had a significant impact on their quality of life.6 If approved, the innovative therapeutic approach of dual Factor XI / XIa inhibition could offer patients the anticoagulation protection they seek with minimal to no risk of bleeding.”
The FDA has also granted abelacimab Fast Track Status for the treatment of thrombosis associated cancer. With clinical trial programs underway for both
atrial fibrillation and Cancer Associated Thrombosis (CAT), abelacimab is the only Factor XI inhibitor being studied for the treatment of arterial and venous thromboembolic events. Abelacimab is an investigational agent and is not approved for any indication in any country.
About Abelacimab
Abelacimab is a novel, highly selective, fully human monoclonal antibody that locks Factor XI in the inactive state, resulting in dual inhibitory activity against both Factor XI and its activated form, Factor XIa.
In patients with atrial fibrillation, abelacimab is planned to be dosed subcutaneously (SC) monthly to maintain nearly complete inhibition in a chronic setting. It is also planned to be administered via an initial intravenous (IV) infusion for acute indications requiring immediate onset of action and then followed by subsequent monthly SC administration. Abelacimab is the only Factor XI inhibitor being studied for the prevention and treatment of both arterial and venous thromboembolic events.
In a PK / PD study, abelacimab administered IV provided profound suppression of Factor XI within one hour after the start of therapy and maintained near maximal inhibition for up to 30 days.7In a Phase 2 study published in the New England Journal of Medicine in 2021, a single intravenous dose of abelacimab after knee surgery reduced the rate of venous thromboembolism by 80%, measured 10 days after surgery, compared to enoxaparin.8 Factor XI inhibition offers the promise of hemostasis-sparing anticoagulation for the prevention and treatment of arterial and venous thromboembolic events.9
Abelacimab received a Fast Track Designation from the FDA in July 2022 for the treatment of thrombosis associated with cancer. In September 2022, abelacimab was also granted a Fast Track Designation for the prevention of stroke and systemic embolism in patients with atrial fibrillation. Abelacimab is an investigational agent and is not approved for any indication in any country.
About Atrial Fibrillation
Atrial fibrillation, or AF, is the most common type of irregular heartbeat. The most severe complication of AF is stroke. The Centers for Disease Control and Prevention (CDC) estimates that 12.1 million people in the United States will have atrial fibrillation by 2030.3 Unfortunately, 40% to 60% of patients with atrial fibrillation are not prescribed anticoagulants today. This underuse of anticoagulants for stroke prevention has been cited as one of the greatest public health issues facing cardiovascular patients.10 In a physician survey, the foremost barrier to patients taking oral anticoagulants was bleed related11.
About the AZALEA-TIMI 71 Phase 2 Study
The AZALEA-TIMI 71 study was an event-driven, randomized, active-controlled, blinded endpoint, parallel-group study with a primary endpoint that evaluates the effect of two blinded doses of abelacimab relative to open-label rivaroxaban in patients with atrial fibrillation (AF) who are at moderate-to-high risk of stroke. The primary endpoint of the AZALEA-TIMI 71 study is the composite of the rate of major or clinically relevant non-major bleeding events. A secondary endpoint is major bleeding on its own. Patients were randomized 1:1:1 and administered subcutaneous (SC) abelacimab 150 mg once-monthly, abelacimab 90 mg once-monthly, or rivaroxaban 20 mg daily. With a median follow-up of 21 months, spanning more than 2,000 patient-years, the AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to a standard-of-care anticoagulant. The study enrolled 1,287 patients across 95 global study sites including the U.S. and Canada, Europe and Asia.
About the LILAC-TIMI 76 Phase 3 Study
The LILAC-TIMI 76 study is an event-driven, randomized, placebo-controlled, double-blind, parallel-group study to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in patients with atrial fibrillation (AF) who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients in the study will be randomized to receive abelacimab 150 mg subcutaneous (SC) or matching placebo once monthly. The study is targeting to enroll approximately 1,900 patients from more than 400 sites in North America, Europe, Latin America, the Middle East and Asia.
About the Abelacimab Phase 3 Program in Cancer Associated Thrombosis (CAT)
The abelacimab Phase 3 CAT program comprises two complementary studies targeting to enroll approximately 2,700 patients across 220 sites in more than 23 countries — the largest program of any anticoagulant performed in cancer-associated thrombosis.
MAGNOLIA is an international multicenter, randomized, open-label, blinded endpoint evaluation, Phase 3 study in patients with gastrointestinal (GI) / genitourinary (GU) cancer in whom DOAC treatment is not recommended. The study will compare the effect of abelacimab relative to dalteparin on VTE recurrence and bleeding in patients with cancer associated VTE who are at a high bleeding risk with non-resectable, locally or regionally invasive GI / GU tumors. Abelacimab 150 mg will be administered intravenously (IV) on Day 1 and subcutaneously (SC) monthly thereafter for up to 6 months; dalteparin administered subcutaneously will be given daily, 200 IU/kg/day for the first month, and then 150 IU/kg/day up to 6 months.
ASTER is an international multicenter, randomized, open-label, blinded endpoint evaluation, Phase 3 study comparing the effect of abelacimab relative to apixaban on venous thromboembolism (VTE) recurrence and bleeding in patients with cancer associated VTE in whom DOAC treatment is recommended. Abelacimab 150 mg will be administered intravenously (IV) on Day 1 and subcutaneously (SC) monthly thereafter for up to 6 months; Apixaban 10 mg will be administered orally, twice daily (bid) for the first 7 days, followed by 5 mg bid up to 6 months.
About Anthos Therapeutics
Anthos Therapeutics was founded by Blackstone Life Sciences in 2019 and obtained from Novartis Pharma the exclusive global rights to develop, manufacture, and commercialize abelacimab. Anthos Therapeutics is a clinical-stage biopharmaceutical company focused on the development and commercialization of genetically and pharmacologically validated innovative therapies to advance care for high-risk cardiovascular patients. For more information, visit the company’s website and follow on Twitter and LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding the initiation, and timing, of future clinical trials and its research and development. All statements, other than statements of historical facts, contained in this press release, including statements regarding the company’s strategy, future operations, future financial position, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “become,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. In addition, the forward-looking statements included in this press release represent the company’s views as of the date hereof and should not be relied upon as representing the company’s views as of any date subsequent to the date hereof. The company anticipates that subsequent events and developments will cause the company’s views to change. However, while the company may elect to update these forward-looking statements at some point in the future, the company specifically disclaims any obligation to do so.
Media Contact:
Caren Begun
TellMed Strategies
908-947-0500 x723
caren.begun@tmstrat.com
